How Many Unpaired Electrons Does Mn Have
5 How many unpaired electrons are present in the ground. Also to know which electrons are most unpaired.

Solved Which Of The Following Ions Has Five Unpaired Electrons A Mathrm Ti 4 B Mathrm Co 2 C Mathrm V 3 D Mathrm Fe 3 E Mathrm Zn 2
Manganese electronic configuration is Ar 3d⁵ 4s².

. Asked Jun 26 2017 in Chemistry by Lola_M. 4 Does P have one unpaired electron. 2 Which element has exactly 2 unpaired electrons in its ground state.
No unpaired electrons therefore diamagnetic. Therefore the d6 tetrahedral high spin complex would have 4 un. In the complex ion ML6n Mn has six d electrons and L is a weak field ligand.
How many electrons are unpaired in a d6 high spin4 unpaired electronsTetrahedral complexes are high spin complex. How many ions does it dissociate into when dissolved in water. The splitting pattern of tetrahedral complex and filling of six electrons when the complex is high spin is shown below.
8 How many electrons are present in the ground state P atom. 5 How many unpaired electrons does CU have in ground state. Also know how many unpaired electrons does the ground state of mn2 have.
Secondly how many unpaired electrons are in Ag. Generally electrons first enter 4s² shell filling. 6 How many unpaired electrons are present in the ground state of P Z 15.
4 Does SN have 2 unpaired electrons in the ground state. 2 paired electrons and 4 unpaired therefore paramagneticAg is d10 system. Ar 4s2 3d5 soMn2 has an electron configuration of.
How many unpaired electrons does Mn have in Mn CN63- ion. What is the coordination number for Co in Mg Co enBr42. Ar 4s0 3d5It looks like that 5 or 3 or at least 1 one electrons are.
Number of unpaired electrons in MnCN_63- is What is the number of unpaired d-electrons which the element Mn can have in the compound. 9 Does P have 3 unpaired electrons. The manganese has 5 unpaired electrons.
The ligands are weak field ligands. How many unpaired electrons does fluorine have - Please explain these problems 1. Is the ion paramagnetic or diamagnetic.
C Te Hf Si. How many unpaired electrons are in the fluorine atom. Which of the following is true for Co OH63-.
1 How Many Of The Following Elements Have 2 Unpaired Electrons In The Ground State. Since Mn3 losses 3 electrons then Mn becomes 3d4 hence it has 4 unpaired electrons. Manganese has 5 electrons in its 3d shell and all five electrons and unpaired maintaining parallel spin since they must obey hunds rule.
3 How many unpaired electrons are there in the ground state of each atom. 7 Does P have two unpaired electrons. Mn is configured.
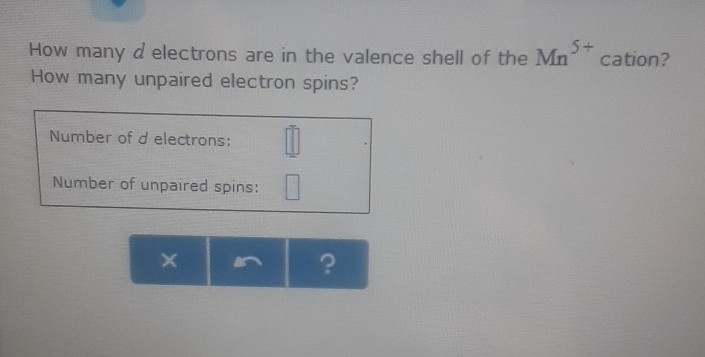
Solved 5 How Many D Electrons Are In The Valence Shell Of Chegg Com

The Hexaquo Manganese Ii Ion Contains Five Unpaired Electrons While The Hexacynoion Contains Only One Unpaired Electron Explain Using Crystal Field Theory From Chemistry Coordination Compounds Class 12 Manipur Board
0 Response to "How Many Unpaired Electrons Does Mn Have"
Post a Comment